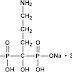Arrow sues to ward off non-patent
 Arrow Generics Ltd and another v Merck & Co Inc [2007] EWHC 1900 (Ch) was a decision of Mr Justice Kitchin in the Patents Court on 31 July. The full text of the judgment is happily available on BAILII.
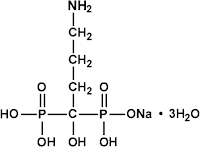
Arrow Generics Ltd and another v Merck & Co Inc [2007] EWHC 1900 (Ch) was a decision of Mr Justice Kitchin in the Patents Court on 31 July. The full text of the judgment is happily available on BAILII.Right: alendronate, which looks like it was invented on a Scrabble board rather than in result of any laboratory research
In July 1998 Merck filed a patent application claiming a priority date of July 1997. The patent (the '292 patent) was granted in November 2001. The trick in this invention was to give osteoporosis patients a single dose 70mg of alendronate once a week rather than 10mg daily, thus producing fewer adverse gastrointestinal events as well as being more convenient for patients. Arrow, a generic drug company, along with two other generic companies, got this patent revoked in January 2003 and then sold lots of 70mg dosages of alendronate themselves, making lots of money.
But had Arrow missed the target? When prosecuting the '292 patent Merck filed four divisional applications for a once-weekly dosage medicament for the treatment of osteoporosis and/or a 70mg preparation of alendronate. Those applications were stayed pending the 292 patent proceedings, but were revived upon their conclusion. To Merck's delight, one of the four (the '904 patent) was granted - designating, among other European Patent Convention (EPC) countries, the United Kingdom.
A letter from the European Patent Office (EPO) on 1 March 2007 confirmed that '904 was being granted said that its decision would take effect on the day on which the mention of grant was published in the European Patent Bulletin, which would be on 28 March. On 22 March Merck faxed the EPO to withdraw the '904 patent's 'GB’ designation. An EPO officer read the fax that very day and, on 4 April, the EPO issued a communique confirming the withdrawal of the patent's ‘Contracting State GB’ status. The EPO added that its 1 March decision to grant the patent would still become effective for the remaining designated states on 28 March and that a corrigendum would be published electronically around two months after publication of the mention of grant.
Left: the IPKat prefers the sword to the Arrow ...
Lewison J was asked to rule on three main issues: (i) had the '904 patent ever been granted; (ii) did section 74 of the Patents Act 1977 - which lists the circumstances in which the validity of a patent may be put at issue - preclude the granting of the declarations sought in relation to the divisional applications and Arrow's own products and (iii) if not, did the court have jurisdiction to make the declarations sought?
Lewison J ruled as follows:
The IPKat says, at para.58 of his judgment the judge explains why the declarations sought by Arrow would serve some purpose:* Although the EPO issued its decision to grant on 1 March 2007, by Article 97(4) of the EPC the grant could not have taken effect until the date on which the mention of the grant was published in the Bulletin. Until that happened, Article 79(3) gave Merck the chance to withdraw the designation of any, or indeed all, contracting states - which Merck did.
* While the Bulletin mentioned the original decision to grant the '904 patent in respect of all the originally designated contracting states, including the UK, that mention didn't mean that a patent governing the UK was granted - because there was never a decision to grant the '904 patent in the UK which could have become effective.
* Since '904 was never granted, the court had no jurisdiction to declare that it was invalid or that it should be revoked. Nor was there any jurisdiction to make declarations as to the validity of the '904 patent in any contracting state in which it had been granted.
* Could Arrow get a declaration that it had the right to make and sell its own products? Yes, since section 74 of the 1977 Act did not bar them from being able to do so.
* Negative declarations should not be granted where they serve no useful purpose. However, if such a declaration helps ensure that the aims of justice are achieved, the court should not be reluctant to grant one.* In this case there was a clearly defined issue which was readily susceptible to determination, and there were no further matters capable of depriving the court of jurisdiction to make the declarations. Accordingly, while the claims relating to the UK bit of '904 would be struck out, the claims relating to the divisional applications and Arrow's own products would be allowed to proceed.
"I begin by considering whether the declarations sought would serve a useful purpose. In my judgment they undoubtedly would. Arrow took proceedings to clear the path before launching its once-weekly 70mg alendronate product – a generic equivalent to Fosamax. It was successful in all those proceedings. But Merck retained a series of divisional applications which also have a "GB" designation. The EPO has now reversed its position and decided, at least for the moment, that Merck is indeed entitled to patent protection for Fosamax and Merck has indicated that it intends to enforce its rights. One divisional application has proceeded to grant but, at the last possible moment, Merck de-designated the UK (for reasons it has not explained) so Arrow cannot apply here to revoke it. Nor can it incur here any liability in respect of it. But the other divisional applications are following on behind and for each of these Merck has retained the "GB" designation. Two of them presently have claims which cover Arrow's product. Despite an invitation, Merck has declined to give Arrow any assurance that it will not seek to enforce its rights arising from those divisionals against Arrow in this country. So Arrow is currently incurring a substantial liability under section 69 of the Act. There is a public interest in commercial certainty in patent matters as in any others. Business needs to know where it stands. I believe this court should assist in providing that certainty where it properly can. The declarations sought would provide Arrow with the assurance that its alendronate product cannot offend against Merck's patent rights arising in this country from the remaining divisional applications. Accordingly, the declarations have a valuable commercial purpose".
Right: skeleton arguments
Merpel adds, section 4(2) of the Patents Act 1977 says:"An invention of a method of treatment of the human or animal body by surgery or therapy or of diagnosis practised on the human or animal body shall not be taken to be capable of industrial application".Why, she says, do I keep getting the feeling that these words have something to do with the treatment of osteoporosis sufferers by giving them a particular dosage of alendronate?
Osteoporosis Education Project here
Recipes for bones here; skin and bones here